Welcome to the COMPETITIVE EXAM MCQs SERIES of ENVIRONMENTAL SCIENCE for UGC-NET/JRF, SLET, GATE, and other entrance tests – Fundamentals of Environmental Chemistry.
Syllabus outline
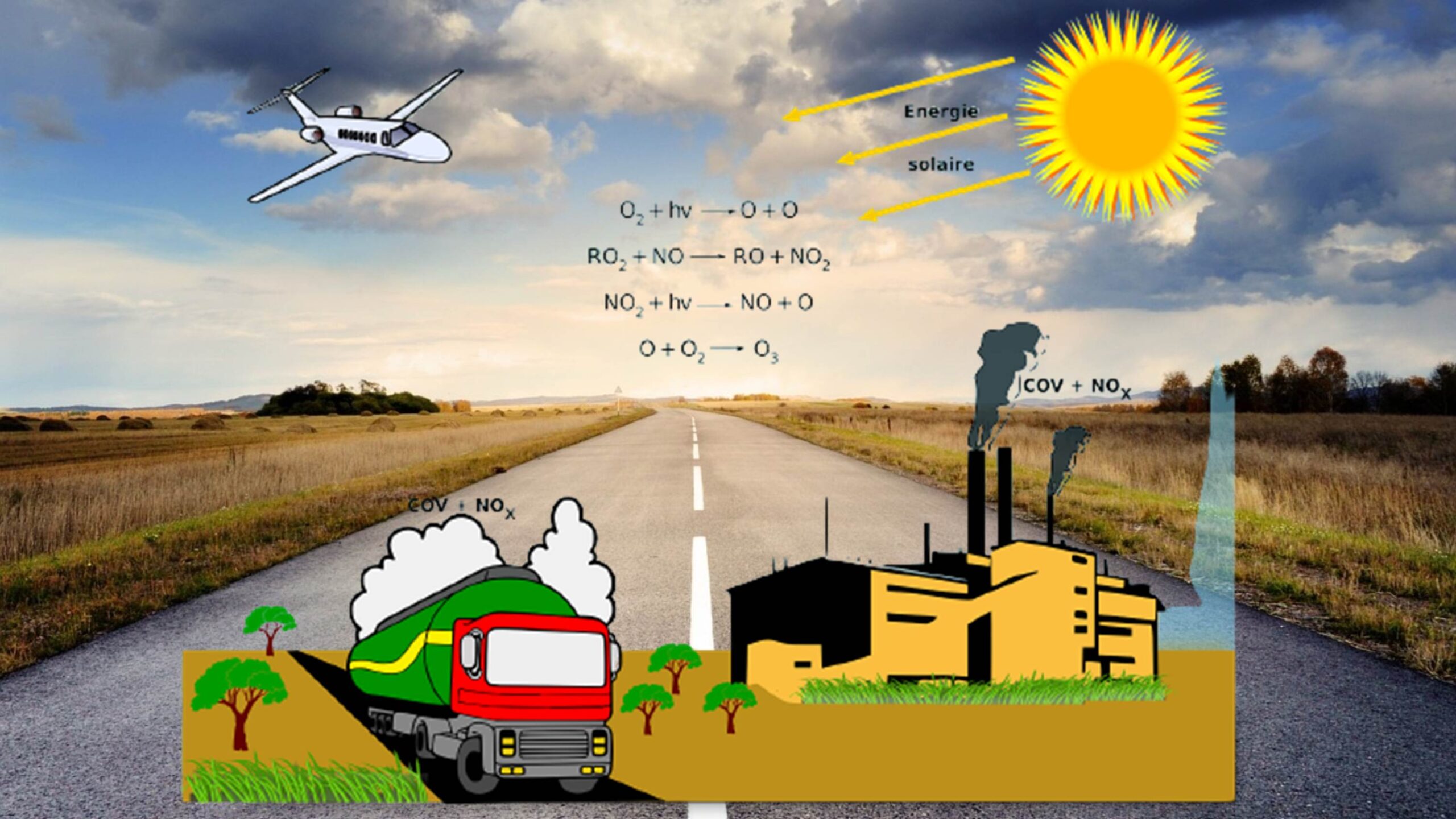
Fundamentals of environmental chemistry encompasses studying the chemical processes occurring in the environment and their impact on the natural world. It also introduces the scope and significance of this field, emphasizing its pivotal role in addressing environmental issues. In addition, understanding the concept of environmental pollution and its far-reaching consequences is a fundamental starting point. It includes introductory comments on the following sub-topics:
- Atmospheric Chemistry
- Water Chemistry
- Soil Chemistry
- Chemistry of Pollutants
- Environmental Analysis
- Green Chemistry
This quiz contains the concept-based most frequently asked 25 MCQs of “Fundamentals of Environmental Chemistry“. Each question has a single correct/most appropriate answer.
1. The term bioavailability refers to:
a) The ease with which a substance can be absorbed and used by living organisms
b) The availability of healthcare in rural areas
c) The quantity of freshwater resources in a region
d) The abundance of plant species in an ecosystem
View Answer2. Which of the following pollutants is a common contaminant in electronic waste (e-waste)?
a) Oxygen
b) Mercury
c) Potassium
d) Carbon dioxide
View Answer3. The Kaya Identity is a formula used to:
a) Assess the economic value of ecosystem services
b) Calculate the concentration of pollutants in the atmosphere
c) Estimate the carbon footprint of a nation
d) Determine the carrying capacity of an ecosystem
View Answer4. What causes the transformation of ice (solid) into water (liquid)?
a) Enhanced particle vibrations
b) Increased kinetic energy accelerating thermal energy
c) Particle collisions
d) Liberation of water molecules after breaking intermolecular bonds
View Answer5. Which pollutant is a major concern in the formation of acid fog in industrial areas?
a) Carbon dioxide
b) Ammonia
c) Nitrogen oxides
d) Sulfur dioxide
View Answer6. A hydrogen atom shared by two water molecules shifts from one molecule to the other which forms:
a) Hydrogen ion and Hydroxide ion
b) Hydrogen peroxide and Hydronium ion
c) Hydrogen ion and Hydronium ion
d) Hydronium ion and Hydroxide ion
View Answer7. Which of the following is a primary source of indoor air pollution in residential homes?
a) Carbon dioxide
b) Radon gas
c) Outdoor air pollution
d) Ground-level ozone
View Answer8. What leads to the atoms of a water molecule having partial charges?
a) Attraction between opposite charges
b) Oxygen donating an electron to hydrogen
c) Hydrogen donating an electron to oxygen
d) Unequal sharing of electrons between the atoms
View Answer9. Which environmental components interface between endogenic and exogenic material cycling?
a) Water
b) Atmosphere
c) Soil and Sediment
d) Living organisms
View Answer10. The phenomenon known as eutrophication occurs when:
a) Oxygen levels in water bodies decrease due to excess nutrients
b) Water bodies experience a sudden increase in salinity
c) Water temperature in lakes and rivers rises significantly
d) Water bodies become more acidic due to pollution
View Answer11. What is the primary reason for the unique properties exhibited by water?
a) The covalent bonding pattern and bond length
b) The ease of ionization of water, even at room temperature
c) The presence of hydrogen bonding between water molecules
d) The bond angle between two hydrogen atoms in a water molecule
View Answer12. When a salt is dissolved in water, each ion is surrounded by a sphere of water molecules which is known as:
a) Micelle
b) Hydration Shell
c) Hydrogen Shell
d) Dehydration Shell
View Answer13. Assertion (A): The pH of rainwater in urban areas is often lower than in rural areas.
Reason (R): Urban areas experience higher emissions of SOx and NOx.
a) Both the A and R are correct, and the R justifies the A.
b) Both the A and R are correct, but the R does not justify the A.
c) The A is correct, but the R is incorrect.
d) Both the A and R are incorrect.
View Answer14. Which of the following pollutants is a major concern in indoor air pollution in homes and workplaces?
a) Carbon dioxide
b) Carbon monoxide
c) Volatile organic compounds
d) Sulfur dioxide
View Answer15. Which of the following pollutants is a major concern in acid mine drainage from abandoned mines?
a) Sulfuric acid
b) Carbon dioxide
c) Nitrogen oxides
d) Sulfur dioxide
View Answer16. The latent heat of the fusion of water is much higher as compared to any other common liquid except:
a) Ammonia
b) Ethanol
c) Hg
d) Fe
View Answer17. Ice floats in liquid water because hydrogen bonds in ice are __________ making ice less dense
a) Compact
b) Mobilized
c) Less in number
d) More ordered
View Answer18. What does redox potential refer to?
a) The concentration of oxygen in the atmosphere
b) The potential for a chemical reaction to release energy
c) The acidity of a solution
d) The ability of a substance to gain or lose electrons in a chemical reaction
View Answer19. Which of the following factors has the greatest influence on the rate of chemical weathering in a given region?
a) Wind speed
b) Precipitation
c) Temperature
d) Humidity
View Answer20. The lower density of ice (solid) compared to water (liquid) is due to _________ in ice.
a) High adhesive force
b) High surface tension
c) Immobilized H bonding
d) Low cohesive force
View Answer21. What would be the pH of a solution with a hydroxide ion concentration of 10^-6 M?
a) pH 10
b) pH 6
c) pH 8
d) pH 2
View Answer22. Which pollutants majorly contribute to ocean acidification?
a) Sulfur dioxide
b) Lead
c) Carbon dioxide
d) Nitrogen oxides
View Answer23. Which pollutant is associated with black carbon or soot particle formation?
a) Carbon dioxide
b) Nitrous oxide
c) Particulate matter
d) Methane
View Answer24. The term carbon offsetting involves:
a) The conversion of carbon to a different chemical form
b) The removal of carbon from the atmosphere by planting trees
c) The release of carbon dioxide into the atmosphere
d) The purchase of carbon credits to compensate for one’s carbon emissions
View Answer25. Which of the following is considered an endocrine-disrupting chemical (EDC) that can affect the hormonal balance in organisms?
a) Silica
b) Phthalates
c) Carbon dioxide
d) Mercury
View AnswerPrevious: Concept of sustainable development
Next: Composition of air
References
- Manahan, Stanley E. (2019) Fundamentals of Environmental Chemistry, CRC Press, 3rd edition.
- Tyagi, Anil K. (2018) Environmental Science and Engineering, Khanna Publishers, 3rd edition.
- Sharma, B. K. (2018) Environmental Chemistry, Goel Publishing House, 2nd edition.

