Phthalate esters (PAEs) are a group of industrial chemicals often present in consumer products as additives. The product includes toys, plastic tableware, clinical devices, and cosmetics. Chemically it is a dialkyl or alkyl aryl ester of phthalic acid i.e. 1,2-benzene dicarboxylic acid. PAEs are present in all environmental compartments worldwide with varying concentrations. These chemicals are added to consumer products to increase their flexibility and durability. PAEs are chronically toxic including Hepatotoxic, Teratogenic & Carcinogenic for human beings and other animals. In addition, intermediate metabolites of PAEs are persistent and toxic. The regulatory norms for the use of phthalate esters are as follows.
Acceptable limits of PAEs
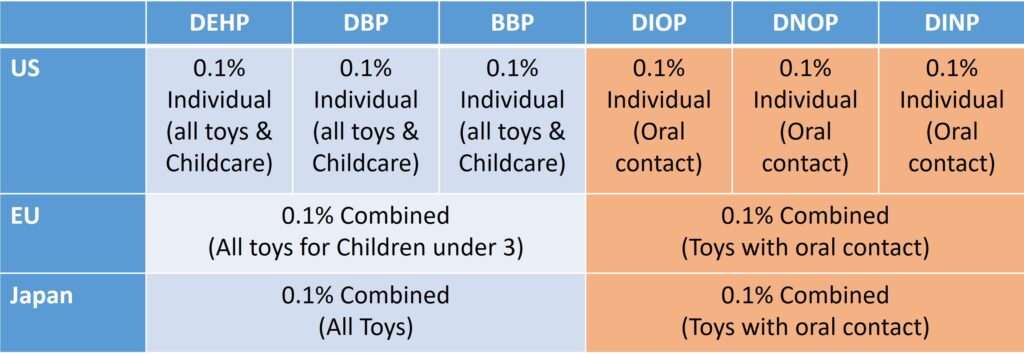
Only a few nations have taken the initiative to restrict the use of PAEs in selected consumer products. The existing regulations are only for children’s toys, childcare articles, and PVC products. However, regulation for PAE use in other polymeric consumer products is missing so far. As per US regulations, all toys and childcare products should not contain more than 0.1% of individual diethyl-hexyl phthalate (DEHP), dibutyl phthalate (DBP), and butyl benzyl phthalate (BBP). In case of oral contact, it should not have more than 0.1% of individual Diisooctyl Phthalate (DIOP), Di-n-octyl phthalate (DNOP), and Diisononyl phthalate (DINP). European Union has defined its regulation considering the cumulative impact of PAEs. As per their guidelines, all toys for children under 3 years should not contain more than 0.1% combined DEHP, DBP, and BBP. In the case of oral contact, it should not contain more than 0.1% combined DIOP, DNOP, and DINP.
Many other countries, including Argentina, Austria, Denmark, Fiji, Finland, France, Germany, Greece, Japan, Mexico, Norway, and Sweden, have implemented similar guidelines. Recently, France banned tubes containing DEHP in pediatric, neonatal, and maternity wards, effective from July 2015.
So far, other developed and developing countries have not taken any steps to regulate the use of PAEs in consumer products. Implementing regulations for PAEs in these countries is essential to protect consumers. Additionally, monitoring PAEs is crucial for properly regulating these widespread pollutants.

