COMPETITIVE EXAM MCQs SERIES of ENVIRONMENTAL SCIENCE for UGC-NET/JRF, SLET, ARS, GATE, and other entrance tests – Environmental Geosciences – Geochemical Classification of Elements.
Syllabus Outline
- Introduction to geochemical classification (e.g. major elements, trace elements, rare earth elements, and isotopes).
- Characteristics and classification of elements (e.g. lithophile, atmophiles, chalcophile, siderophile, and biophilic).
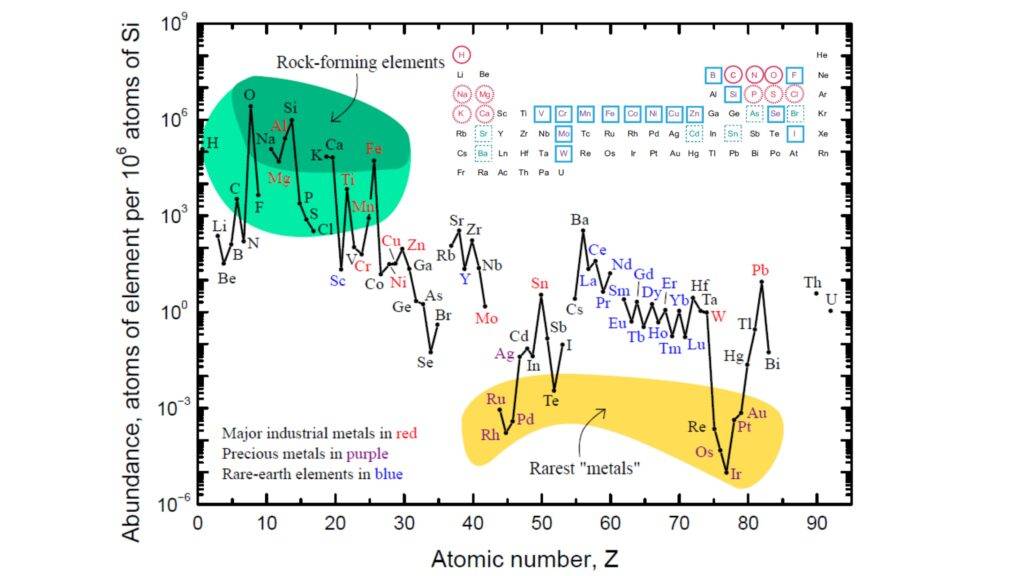
- Composition and distribution of major elements in Earth’s crust.
- Role of silicate minerals in major element classification and the environmental significance of oxides, carbonates and sulfides.
- Behaviour and distribution of trace and rare Earth elements in geological systems.
- Factors influencing mobility and concentration of elements.
- Principles and applications of isotope geochemistry.
- Geochemical modelling and also ethical considerations in environmental geochemistry.
This quiz contains the concept-based most frequently asked 25 MCQs of “Environmental Geosciences – Geochemical Classification of Elements“. Each question has a single correct/most appropriate answer.
*****
1. Chalcophile elements are separated below lithophile elements during:
a) Crystallization of the Earth’s core
b) Volcanic eruptions
c) Differentiation of the Earth’s crust
d) Formation of the mantle
2. Which geochemical classification includes elements having a strong affinity for combining with oxygen?
a) Lithophile
b) Siderophile
c) Atmophile
d) Chalcophile
3. The partitioning of elements between minerals and melts during crystallization is governed by which principle?
a) Raoult’s Law
b) Bowen’s Reaction Series
c) Goldschmidt’s Rules
d) Hess’s Law
4. Which elements are classified as organophiles due to their association with organic compounds in biochemical and biogeochemical processes?
a) C, H, N
b) Cu, Pb, Zn
c) Re, Os, W
d) Be, Mg, Si
5. The distribution of which element is often used to trace the extent of hydrothermal alteration in mineral deposits?
a) Zinc
b) Copper
c) Sodium
d) Lead
6. The bioaccumulation concept of elements in aquatic ecosystems primarily refers to the accumulation of which type of elements?
a) Noble Gases
b) Halogens
c) Transition Metals
d) Alkaline Earth Metals
7. Which elements in Goldschmidt’s classification are preferentially partitioned into silicate minerals?
a) Atmophile
b) Lithophile
c) Chalcophile
d) Siderophile
8. Lithophile elements are concentrated in which portion of the Earth?
a) Mantle
b) Hydrosphere
c) Crust
d) Core
9. Lithophile elements are difficult to reduce to the elementary state due to their strong affinity for:
a) Sulfur
b) Carbon
c) Oxygen
d) Nitrogen
10. Which of the following elements is typically classified as a trace element in geochemistry?
a) Zinc
b) Silicon
c) Calcium
d) Iron
11. Which group of elements is depleted in the silicate portion of the Earth and concentrated in the metallic core?
a) Atmophile
b) Lithophile
c) Chalcophile
d) Siderophile
12. What is the primary control on the distribution of rare earth elements in geological systems?
a) Weathering processes
b) Temperature gradients
c) Organic matter content
d) Crystal structure
13. Which classification system is primarily used to describe the temperature interval over which elements condense from a gaseous state to a solid or liquid state during nebular condensation and planetary accretion?
a) Metallic Behavior Classifications
b) Cosmochemical Classifications
c) Refractory Classifications
d) Partitioning Classifications
14. What is used to describe elements that tend to accumulate in the Earth’s core due to their high density?
a) Chalcophile
b) Lithophile
c) Atmophile
d) Siderophile
15. Which of the following elements is commonly associated with the formation of acid mine drainage?
a) Titanium
b) Chromium
c) Iron
d) Nickel
16. Which element is proposed as a biophile element in the recent study but not traditionally classified as such?
a) Copper
b) Zinc
c) Arsenic
d) Iron
17. Which element is commonly used as a redox condition indicator in the Earth’s crust?
a) Potassium
b) Calcium
c) Sodium
d) Manganese
18. The isotopic fractionation concept in geochemistry refers to the preferential partitioning of isotopes of which element during physical or chemical processes.
a) Carbon
b) Oxygen
c) Hydrogen
d) Nitrogen
19. The concept of compatibility of elements in mineral structures is primarily based on their relative abundance in which geological feature?
a) Igneous rocks
b) Metamorphic rocks
c) Hydrothermal veins
d) Sedimentary rocks
20. Goldschmidt classification is primarily based on the affinity of elements for which of the following?
a) Volcanic eruptions
b) Formation of minerals
c) Atmospheric composition
d) Biological processes
21. Which classification includes elements that can be lithophile, organophile, chalcophile, and siderophile, depending on oxidation state and chemical environment?
a) Partitioning Classifications
b) Cosmochemical Classifications
c) Metallic Behavior Classifications
d) Refractory Classifications
22. Which classification includes elements that commonly form sulfide-type minerals?
a) Chalcophile
b) Siderophile
c) Organophile
d) Atmophile
23. Which classification includes elements that readily form volatile compounds at relatively low temperatures and are preferentially concentrated in planetary atmospheres?
a) Siderophile
b) Atmophile
c) Chalcophile
d) Organophile
24. Which element is considered a major component of the Earth’s crust and is abundant in silicate minerals?
a) Carbon
b) Aluminum
c) Sodium
d) Oxygen
25. The elements that are preferentially partitioned into the metallic core are known as:
a) Siderophile
b) Chalcophile
c) Atmophile
d) Lithophile
*****
Previous: Earth’s Thermal Environment and Seasons
Next: Hydrogeology and Hydrology
References
- Drever, J.I. (1997). Geochemistry of Natural Waters: Surface and Groundwater Environments. Oxford University Press. 1st Edition.
- White, A.F., and Stallard, R.F. (2013). Geochemistry. John Wiley & Sons. 1st Edition.
- Faure, G., and Mensing, T.M. (2005). Isotopes: Principles and Applications. John Wiley & Sons. 3rd Edition.
- Langmuir, D. (1997). Aqueous Environmental Geochemistry. Prentice Hall. 1st Edition.