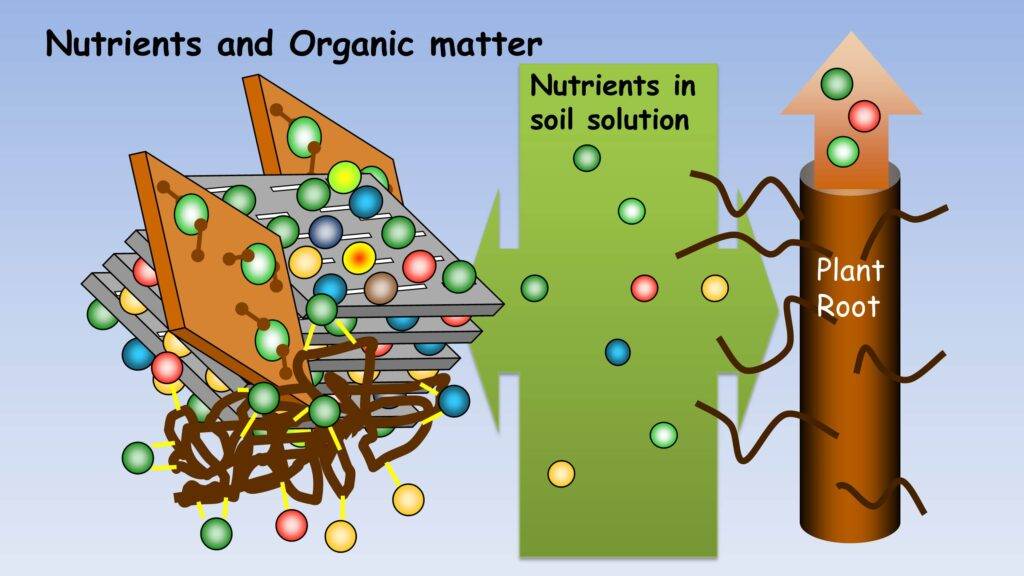
We’re here to tackle the nitty-gritty of Environmental Science, specifically focusing on inorganic and organic components of soils. This series is for UGC-NET/JRF, SLET, GATE, and more.
Syllabus outline
- Minerals and Mineralogy of Soil and Soil Particles (e.g. Sand, Silt, Clay).
- Soil pH, Acidity and Cation Exchange Capacity.
- Nutrient Cycles (e.g. Nitrogen, Phosphorus, Potassium)
- Soil Organic Matter and Humus and its Role.
- Decomposition Processes, Soil Microorganisms, Organic Carbon and its Measurement
- Soil Contamination and Remediation – Heavy Metals, Pesticides and Herbicides and Remediation Techniques.
- Methods for Soil Analysis, Interpretation of Soil Test Results and their Importance in Agricultural Practices
- Soil-Plant Relationships, impact on Water Quality and Soil as a Carbon Sink
We’ve handpicked 25 questions on the fundamentals of “Inorganic and organic components of soils”. It’s not your average quiz; it’s a concept-based exploration into the depths of soil science.
Get ready for a brain workout:
1. What is the primary role of organic material in soil?
a) Affecting the mechanisms by which nutrients get into plant roots
b) Acting as a source of energy for soil microorganisms
c) Enhancing soil pH levels
d) Providing micronutrients to plants
2. Soil cation exchange capacity decreases as
a) The soil surface area decreases
b) The amount of organic matter increases
c) The amount of clay increases
d) The soil pH increases
3. What is the main function of soil organic matter in retaining nutrients?
a) Cation exchange capacity
b) Water retention
c) Soil compaction
d) pH adjustment
4. Which element is a micronutrient essential for plant growth and is often deficient in soils?
a) Nitrogen
b) Iron
c) Phosphorus
d) Carbon
5. What is the approximate composition of loamy soil?
a) 20% sand, 30% silt, 50% clay
b) 40% sand, 40% silt, 20% clay
c) 30% sand, 40% silt, 30% clay
d) 50% sand, 30% silt, 20% clay
6. How can measurements of the percentages of sand, silt, and clay in a soil be used to determine the soil type in a field or garden?
a) By using the soil triangle diagram
b) By conducting a chemical analysis of the soil
c) By measuring the soil’s pH levels
d) By observing the colour of the soil
7. Which of the following statements is incorrect about soil?
a) If nutrients remained in solution they could all be quickly lost from the soil.
b) Soil solution is a complex solution containing many nutrients, trace elements and complex organic molecules.
c) Absorption refers to the ability of an object to attract and hold particles on its surface
d) Plant roots can readily take up nutrients in the soil solution
8. The organic component in soil that mainly serves as a source of energy for soil organisms is:
a) Clay
b) Lignin
c) Quartz
d) Cellulose
9. Organic matter in soil is primarily composed of:
a) Iron and aluminium oxides
b) Carbonates and sulfates
c) Silica and clay
d) Cellulose and lignin
10. The mineral responsible for the cation exchange capacity in soils is:
a) Graphite
b) Montmorillonite
c) Feldspar
d) Gypsum
11. Which inorganic component in soil can contribute to poor drainage and aeration?
a) Sand
b) Silt
c) Clay
d) Gravel
12. What is the primary role of organic matter in soil?
a) To increase soil compaction
b) To provide nutrients to plants
c) To maintain soil pH
d) To retain water
13. Which element is the most abundant in Earth’s crust?
a) Silicon
b) Iron
c) Calcium
d) Oxygen
14. How are the layers of illite clay held together?
a) Fairly weak bonds
b) Strong bonding due to the presence of positively charged potassium ions
c) Strong bonding due to the presence of positively charged calcium and sodium ions
d) They are not composed of layers
15. Which inorganic component in the soil is mainly responsible for retaining and releasing essential plant nutrients?
a) Sand
b) Clay
c) Gravel
d) Silt
16. What are clays primarily composed of?
a) Hydrous aluminium silicates
b) Sodium chloride
c) Calcium carbonate
d) Silicon dioxide
17. Which of the following is NOT a type of clay mineral?
a) Limestone
b) Illite
c) Montmorillonite
d) Kaolinite
18. What is the main source of inorganic components in soils?
a) Atmospheric deposition
b) Volcanic eruptions
c) Plant decomposition
d) Human activities
19. Which organic component of soil resists decomposition and contributes to soil humus?
a) Starch
b) Lignin
c) Cellulose
d) Glucose
20. Which of the following components may contribute to soil compaction?
a) Organic matter
b) Silt
c) Clay
d) Sand
21. Which inorganic component in soil contributes to soil acidity?
a) Aluminum
b) Limestone
c) Gypsum
d) Organic matter
22. The inorganic component responsible for the brown colour of many soils is:
a) Iron oxides
b) Aluminum oxides
c) Calcium carbonate
d) Silica
23. What inorganic component in the soil can enhance its water-holding capacity?
a) Sand
b) Gravel
c) Clay
d) Silt
24. The term cation exchange capacity refers to the soil’s ability to:
a) Retain negatively charged ions
b) Retain positively charged ions
c) Retain both negatively and positively charged ions
d) Neutralize soil pH
25. Which inorganic component of soil is essential for plant photosynthesis and is often deficient in acidic soils?
a) Calcium
b) Magnesium
c) Phosphorus
d) Potassium
Previous: Water as a universal solvent
Next: Biogeochemical cycles
References
- Manahan, Stanley E. (2019) Environmental Chemistry, CRC Press, 10th edition.
- Tyagi, Anil K. (2018) Environmental Science and Engineering, Khanna Publishers, 3rd edition.
- Reddy, M. Anji (2016) Principles of Environmental Chemistry, BS Publications, 4th edition.
- Singh, A. K. (2017) Environmental Chemistry, New Age International Publishers, 2nd edition.
