Water is the elixir of life on Earth encoded in its simple molecule composed of two hydrogens and one oxygen atom. Moreover, it indeed exhibits numerous divine and unique properties that intertwine with its structure. The remarkable properties of water have a profound impact on various natural processes and phenomena, influencing everything from climate regulation to the survival of living organisms. There are several novel properties and facts that are not widely known. Here are some lesser-known 21 divine and exceptional properties of water and facts.
1. Central to life on Earth
Water is essential for all known forms of life on Earth. Its abundance and ability to dissolve various substances make it a crucial medium for biochemical reactions. Moreover, water’s unique properties make it an ideal medium for biochemical reactions, making it essential for the functioning of cells and the sustenance of life. In addition, water is essential for hydration and maintaining bodily functions in all living organisms.
Water has a high specific heat capacity, meaning it can absorb and retain significant amounts of heat without undergoing rapid temperature changes. This property helps in regulating the Earth’s climate and stabilizes body temperatures in organisms. In addition, the hydrogen bonds in water make it an excellent temperature buffer, helping to moderate Earth’s climate and maintaining stable temperatures in aquatic environments.
Water’s prevalence in all living beings and its essential role in sustaining life has led to its association with divine connectivity among all forms of existence.
2. Unique Dissolving Ability
Water’s molecular structure enables it to dissolve a wide range of substances, earning it the title of “universal solvent.” It can dissolve any polar and/or ionic compounds irrespective of the size of the molecules. Due to its polar nature, water can form hydrogen bonds with other substances, allowing it to dissolve a wide range of solutes. This property facilitates the transport of nutrients and waste products within living organisms. Water’s ability to dissolve and dilute substances aid in self-cleaning processes in nature, such as natural purification in rivers and lakes. This ability of water to dissolve a large number of molecules is because of its polar structure along with its small molecular size.
3. Very high Cohesion and Adhesion
The highly cohesive and adhesive nature of water is primarily due to its polar molecular structure, which allows for the formation of strong hydrogen bonds between water molecules and between water and other materials. Therefore, it creates a strong surface tension. Pure water has the highest surface tension (72 dynes/cm at room temperature) after mercury. These properties are vital in numerous natural processes and have significant biological implications such as the formation of droplets and helping plants to draw water from their roots to their leaves. In addition, water molecules can adhere to other surfaces, promoting capillary action in plants, which aids in the movement of water against gravity.
5. High Surface Tension
Water has high surface tension due to the unique properties of its molecules and the forces between them. Surface tension arises from the cohesive forces between water molecules at the liquid-air interface. These cohesive forces are primarily a result of hydrogen bonding, which is the attraction between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule. The hydrogen bonding allows water molecules to stick together, creating a strong network at the surface.
There are several environmental and biological significances of water’s high surface tension. For example, the formation of droplets, facilitating capillary action in plants, reducing evaporation rates, and helping in many cellular processes. In addition, this property allows insects like water striders to walk on water.
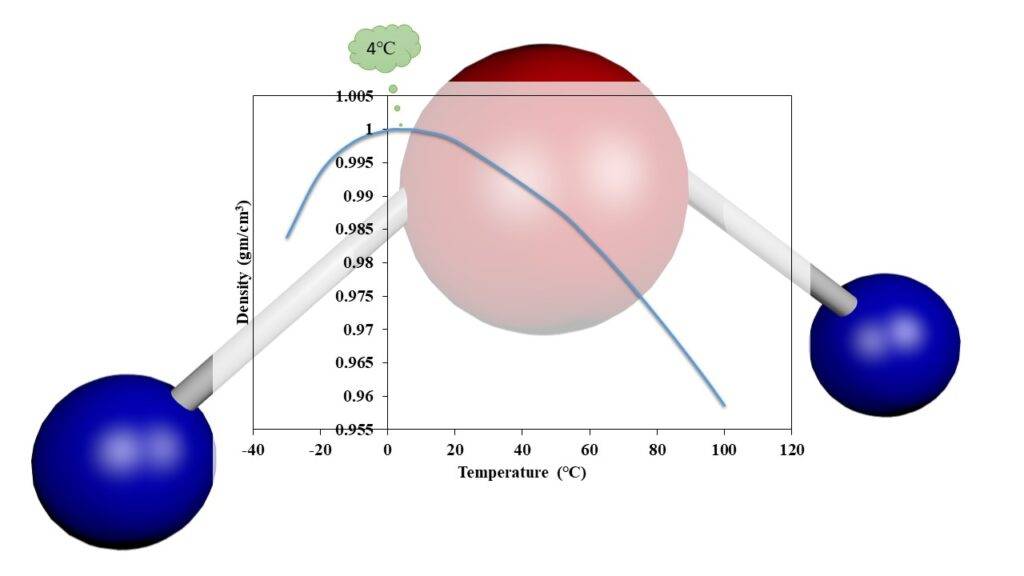
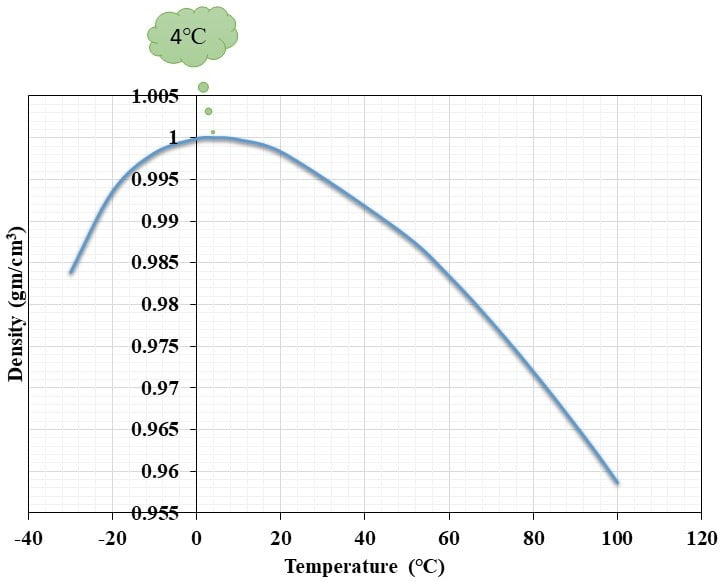
4. Negative Thermal Expansion
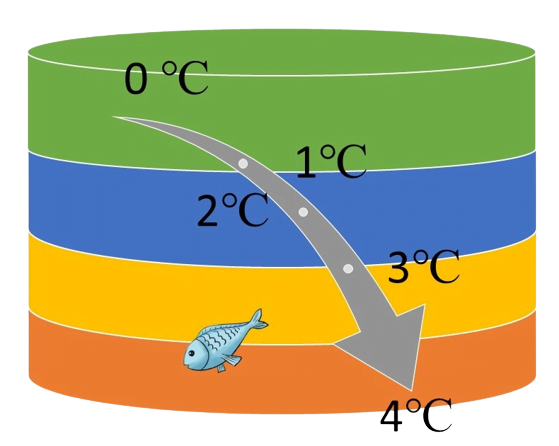
Unlike most substances, which expand when heated, water exhibits negative thermal expansion between 0°C and 4°C. As it warms up in this range, it actually contracts, making it denser and contributing to its maximum density at 4°C. Furthermore, it expands as it freezes. Most substances become denser as they cool and solidify, but water is an exception
This property is crucial for life, as ice forms a layer on the surface of water bodies, insulating the underlying water and allowing life to persist during cold periods. Furthermore, this property is employed in the process of rock weathering, contributing to the formation of soil. Liquid water enters the fissures of rocks and expands upon freezing, thereby promoting the weathering of rocks.
Negative thermal expansion is possible due to the formation of a hexagonal lattice structure in ice with each water molecule bonded to four neighboring molecules. This hexagonal arrangement is responsible for the unique properties of ice and is essential for supporting life in cold environments.
6. Transparency and Reflective
Water is transparent to visible light, allowing sunlight to penetrate aquatic ecosystems and support photosynthesis, which forms the basis of the food chain.
This is because of the small size and unique arrangement of two hydrogen atoms bonded to one oxygen atom. This molecular structure results in a relatively symmetrical distribution of positive and negative charges within the molecule. As a result, water does not strongly absorb visible light wavelengths, which are responsible for the colors we perceive. Instead, most of the visible light passes through water without being absorbed or scattered significantly.
In addition, the water’s surface acts as a mirror, reflecting light and natural surroundings, providing an aesthetically pleasing aspect of nature.
7. Guidance and navigation
Bodies of water, such as rivers guide travelers, leading them towards their destinations. In addition, the water flow patterns are often used by animals to navigate their destination or search for food and shelter.
8. Quantum Entanglement
At the quantum level, water molecules can exhibit quantum entanglement, a phenomenon where particles become interconnected regardless of distance. This aspect, still a subject of intense scientific exploration, may hold implications for understanding deeper aspects of reality and interconnectedness.
9. Destruction and Renewal
Water’s role in natural disasters like floods and its subsequent contribution to renewal through nourishing the land is significant for the environment. Although this divine power of water has negative short-term effects, it is important for the planet as a whole.
10. Malleability
Water’s ability to erode rocks and shape landscapes over time is a representation of its divine influence in shaping the Earth’s features.
11. Sound Transmission
Sound travels efficiently through the water, making it a vital environment for marine animals that rely on sound for communication and navigation.
12. Piezoelectric Effect
Certain crystalline structures within ice can exhibit the piezoelectric effect, where mechanical stress generates electric charges. This property has relevance in understanding the energetics of water and how it interacts with its environment.
13. Hydroelectric Energy
Water’s movement and flow can be harnessed to generate hydroelectric energy, a renewable and sustainable energy source. This aspect underscores water’s potential as a source of divine and life-sustaining energy.
14. Medium for Energy Transfer
Water’s high heat capacity and thermal conductivity make it an excellent medium for transferring and distributing energy, be it in cooling systems or natural processes like ocean currents regulating Earth’s climate.
Since water has a high specific heat capacity, which means it can absorb a large amount of heat energy without undergoing a significant change in temperature. This property helps to regulate Earth’s climate by absorbing heat from the sun during the day and releasing it slowly at night, moderating temperature fluctuations in coastal regions.
Water’s high heat capacity also contributes to the thermal stability of aquatic ecosystems. Bodies of water can store large amounts of heat, acting as a buffer against extreme temperature changes. This stability provides a suitable environment for various aquatic life forms.
15. Quantum Coherence in Photosynthesis
Some research suggests that quantum coherence in water molecules might play a role in optimizing energy transfer during photosynthesis in plants, making this life-giving process even more efficient. Therefore, this property has been listed among the 21 divine properties of water. However, it’s essential to clarify that the existence and significance of quantum coherence in water are still topics of ongoing research and debate. The scientific understanding of this phenomenon is not yet fully established.
16. Resonance and Vibration
Water can resonate and exhibit specific vibration patterns, which have been linked to the concept of “vibrational healing” and its potential effects on living organisms. Although this has not been well-studied, it has the potential to change living organisms and the surrounding environment. Therefore, the resonance and vibration of water molecules have been included in 21 divine properties of water.
17. Water’s Role in Homeostasis
Water plays a crucial role in maintaining the body’s homeostasis, regulating temperature, transporting nutrients, and removing waste. This is possible because water has high specific heat as compared to any other comparable liquids. The ability of water to store and release energy is essential for sustaining life processes.
18. Water as a Connector of Ecosystems
Bodies of water serve as vital connectors between diverse ecosystems, fostering biodiversity and reinforcing the interconnectedness of life forms. Since the ecological processes can not be possible without water involvement. Therefore, it is highly significant for all life as we see it on the planet.
19. Blue Color Absorption
While water appears colorless in small quantities, it does absorb light selectively in the red part of the visible spectrum. When sunlight penetrates deep water, the water absorbs the longer-wavelength red light, giving it a blue hue. This phenomenon significantly contributes to the blue appearance of Earth when observed from space.
20. High Refractive Index
Water has a relatively high refractive index, which causes light to bend when it passes through water, leading to various optical effects, such as the apparent bending of objects when viewed through water. This is important to establish a balanced pray-predator relationship between aquatic and terrestrial animals. Therefore, the refractive index has been included in 21 divine properties of water.
21. Self-Ionization and pH regulation
Pure water is not completely neutral but undergoes self-ionization, where a small fraction of water molecules dissociates into hydrogen ions (H+) and hydroxide ions (OH–). This self-ionization plays a crucial role in acid-base chemistry and pH regulation in both living organisms and natural environments.
Conclusion
The above-discussed 21 divine and unique properties of water make it an indispensable component of life on Earth, shaping our planet in countless ways. As the universal solvent, thermal regulator, and support system for various ecosystems, water’s influence reaches far beyond its deceptively simple chemical structure. Understanding and preserving this precious resource is vital for maintaining the delicate balance of life on our blue planet.

