COMPETITIVE EXAM MCQs SERIES of LIFE SCIENCES for UGC-CSIR NET/JRF, SLET, GATE, and other entrance tests – MOLECULES AND THEIR INTERACTION RELEVANT TO BIOLOGY – Enzyme Kinetics and Catalysis Basics.
Syllabus Outline
- General principles of catalysis – activation energy, transition state
- Enzyme-substrate interaction models – lock and key, induced fit
- Michaelis-Menten kinetics – Vmax, Km, Lineweaver-Burk plot
- Types of enzyme inhibition: competitive, non-competitive, and uncompetitive
- Allosteric enzymes and sigmoidal kinetics
- Feedback regulation and covalent modification
- Mechanisms of catalysis – acid-base, covalent, metal ion catalysis
- Role of cofactors and coenzymes
- Isozymes – structure, tissue-specific roles, diagnostic importance
This quiz contains concept-based, most frequently asked 25 MCQs of “MOLECULES AND THEIR INTERACTION RELEVANT TO BIOLOGY – Enzyme Kinetics and Catalysis Basics”. Each question has a single correct/most appropriate answer.
*****
1. Assertion (A): Enzymes increase the rate of a reaction by stabilising the transition state relative to the substrate.
Reason (R): Enzymes lower the activation energy equally for both the forward and reverse reactions, ensuring that the equilibrium constant (Keq) remains unchanged.
A) Both A and R are true, and R correctly explains A.
B) Both A and R are true, but R does not correctly explain A.
C) A is true, but R is false.
D) A is false, but R is true.
2. An enzyme accelerates a reaction rate by a factor of 10⁷. A transition-state analogue is synthesised for this reaction. Assuming optimal stabilisation, what is the expected ratio of the dissociation constant (Ki) of the transition-state analogue relative to the Michaelis constant (Km) of the substrate?
A) Ki ≈ Km × 10⁷
B) Ki ≈ Km
C) Ki ≈ Km / 10⁷
D) Ki is unrelated to Km
3. According to the Lock-and-Key model, the enzyme’s active site is:
A) Flexible and changes conformation upon binding
B) Requires multiple subunits for binding
C) Complementary only to the transition state
D) Rigid and pre-shaped, complementary to the substrate
4. An enzyme exhibits sigmoidal kinetics in a V0 vs [S] plot. This indicates:
A) Covalent modification
B) Competitive inhibition
C) Cooperative substrate binding
D) Ping-pong mechanism
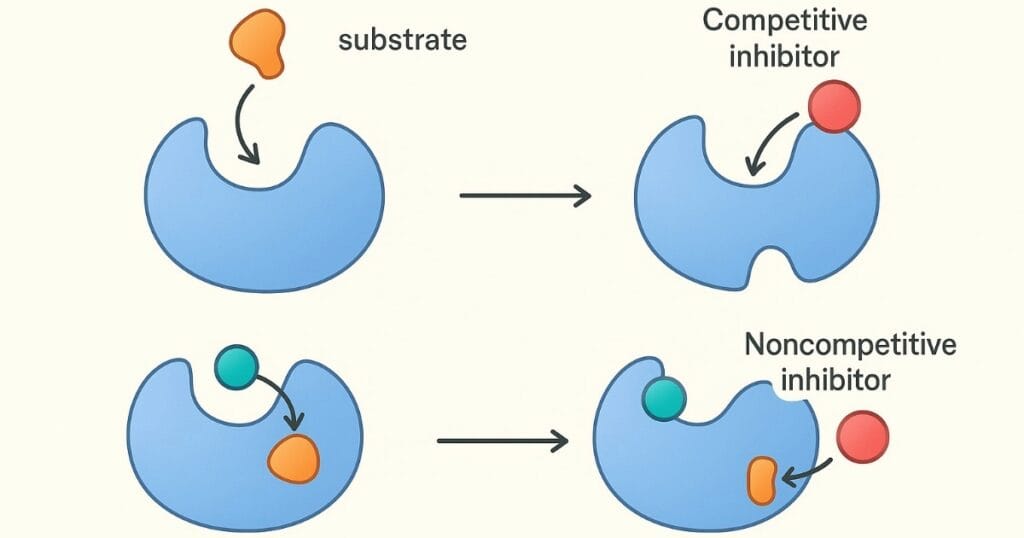
5. Competitive inhibition affects enzyme kinetics by:
A) Decreasing Vmax, no change in Km
B) Increasing Km, Vmax unchanged
C) Decreasing both Km and Vmax
D) Increasing both Km and Vmax
6. In the Lineweaver–Burk plot, uncompetitive inhibition produces:
A) Lines intersecting on the x-axis
B) Parallel lines
C) Intersection on the y-axis
D) Single line
7. Noncompetitive inhibition is characterised by:
A) Inhibitor binds only to the free enzyme
B) Inhibitor binds only to ES
C) Inhibitor binds irreversibly
D) Inhibitor binds equally well to E and ES
8. Mechanism-based inhibitors are also called:
A) Transition-state analogues
B) Allosteric inhibitors
C) Noncompetitive inhibitors
D) Suicide inhibitors
9. In a Lineweaver–Burk plot, the intersection left of the y-axis represents:
A) Mixed inhibition
B) Competitive inhibition
C) Uncompetitive inhibition
D) Noncompetitive inhibition
10. Histidine in enzyme active sites often acts as:
A) Metal ion stabiliser
B) Proton acceptor
C) Proton donor
D) Both acid and base catalyst
11. The correct sequence for chymotrypsin catalysis:
A) Substrate binding → acylation → deacylation → product release
B) Acylation → substrate binding → hydrolysis → deacylation → product release
C) Deacylation → acylation → substrate binding → product release
D) Hydrolysis → acylation → deacylation → product release
12. Biotin acts as a coenzyme for the transfer of:
A) Acyl groups
B) CO₂
C) Methyl groups
D) Phosphate groups
13. Proximity and orientation effects:
A) Lower activation energy by entropy reduction
B) Increase ΔG°
C) Affect Vmax and Km both
D) Depend on metal cofactors
14. Ping-pong mechanism involves:
A) Enzyme alternates between two forms
B) Two substrates bound simultaneously
C) Sequential binding of two substrates
D) Same enzyme and substrate system produces two different products in the presence of uncompetitive inhibitors
15. Hill coefficient (nH) > 1 indicates:
A) Negative cooperativity
B) Positive cooperativity
C) No cooperativity
D) Allosteric inhibition
16. According to the Monod-Wyman-Changeux model of allosteric regulation, an allosteric activator:
A) Shifts the equilibrium toward the tense state
B) Shifts the equilibrium toward the relaxed state
C) Inhibits catalytic activity
D) Decreases substrate binding affinity
17. According to the Koshland-Némethy-Filmer model of allosteric regulation, cooperativity arises through:
A) Induced-fit conformational changes upon ligand binding
B) A pre-existing equilibrium between tense and relaxed states
C) Covalent modification of enzyme subunits
D) Subunit dissociation and reassembly
18. Allosteric enzymes show which property?
A) Hyperbolic kinetics
B) Michaelis–Menten behaviour
C) Sigmoidal kinetics
D) Single-subunit activity
19. If Hill coefficient = 4, it suggests:
A) No cooperativity
B) Noncompetitive cooperativity
C) Negative cooperativity
D) Perfect concerted cooperativity
20. At high substrate concentration, the isoenzyme with a higher Vmax is favoured because:
A) The reaction rate becomes independent of substrate concentration and depends only on kcat.
B) The enzyme’s high Vmax allows faster substrate binding at high concentrations.
C) Product formation is limited by feedback inhibition rather than turnover number.
D) The enzyme exhibits cooperative binding behaviour at high substrate concentration in the case of higher Vmax.
21. In muscular dystrophy, the level of the LDH₅ (lactate dehydrogenase isoenzyme 5) isoform:
A) Increases
B) Decreases
C) Remains unchanged
D) Is inhibited by ATP
22. Proteolytic activation (zymogen activation) is:
A) Reversible
B) Irreversible
C) Allosteric
D) Feedback controlled
23. Assertion (A): An uncompetitive inhibitor increases the apparent Hill coefficient and shifts the sigmoidal curve to the left.
Reason (R): Uncompetitive inhibition stabilises the ES complex, promoting R-state transition.
A) Both A and R are true, and R correctly explains A.
B) Both A and R are true, but R does not correctly explain A.
C) A is true, but R is false.
D) A is false, but R is true.
24. Kinetic isotope effect studies are useful to:
A) Measure diffusion rates
B) Study mixed inhibition
C) Determine enzyme mass
D) Identify rate-determining steps
25. Transition-state analogues act as:
A) Potent inhibitors that mimic the geometry and charge distribution of the transition state.
B) Substrate mimics that bind loosely and are readily displaced by the natural substrate.
C) Allosteric activators that enhance enzyme activity through conformational changes.
D) Reversible inhibitors that bind weakly to the enzyme’s active site.
*****
Previous: Bioenergetics and Energy Pathways
Next: Protein Folding and Structure Guide
References
- Nelson, David L. & Cox, Michael M. (2021). Lehninger Principles of Biochemistry, W. H. Freeman, 8th Edition
- Voet, Donald, Voet, Judith G., & Pratt, Charlotte W. (2018). Voet’s Principles of Biochemistry, Wiley, 5th Edition
- Berg, Jeremy M., Tymoczko, John L., & Stryer, Lubert (2023). Biochemistry, W. H. Freeman, 10th Edition
- Palmer, Trevor & Bonner, Philip L. (2007). Enzymes: Biochemistry, Biotechnology, Clinical Chemistry, Horwood Publishing, 2nd Edition
- Upadhyay, Avinash, Upadhyay, K., & Nath, Nirmalendu (2023). Biophysical Chemistry: Principles and Techniques, Himalaya Publishing House, 4th Edition
🔗 Explore More MCQs: